About the Study
The Op NON-STOP study is currently recruiting. See below for the latest enrolment figures and other key information about the trial.
Information About the Study
The aim of this study is to evaluate the clinical and cost-effectiveness of containment surgery compared to best conservative care amongst children aged 5 to 12 years old with Perthes’ Disease.
-

TREATMENT
In the Op NON-STOP Study (Operative or Non-Surgical Treatment of Perthes’ disease) half of the children will receive ‘active containment’ and half will receive ‘surgical containment’.
-

THINGS TO KNOW
At the end of the study, it will help everyone to know what the best treatment is. To make sure people learn about the best treatment, the doctors who help with this study will talk to other doctors, and other people in the NHS who write national guidelines.
-

PERSONAL DATA
Personal details will be held by the research team at the University of Oxford. All information that is collected during the course of the research will be used and stored in accordance with the current data protection legislation.
-

STUDY FUNDER
This study is funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (NIHR152309).
-

STUDY SPONSOR
Alder Hey Children’s NHS Foundation Trust is the sponsor for Op NON-STOP.
-

STUDY DESIGN
Op NON-STOP is a multi- centre prospective randomised superiority trial.
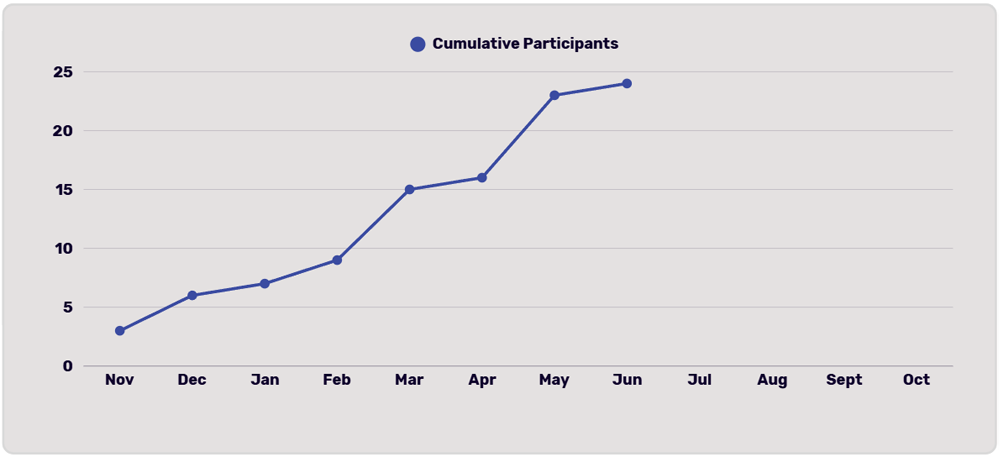
Recruitment Chart
Study Team
-

PROF DAN PERRY
Chief Investigator
Dan is an NIHR Research Professor and a consultant in children's orthopaedic surgery.
He leads international clinical research in a broad array of conditions & injuries that affect children, and loves to ensure that research is presented in a way that is accessible to everyone. -

MR NICK NICOLAOU
Joint Lead Grant Applicant
Nick is a Consultant Paediatric Orthopaedic Surgeon at Sheffield Children’s Hospital.
He is a founding member of the British Society for Children’s Orthopaedic Surgery (BSCOS), research committee and an opinion leader within BSCOS. -

LOUISE SPOORS
Study Manager
Louise manages a suite of children’s trauma trials at the University of Oxford.
She was a nurse for many years and has a real understanding of the challenges of doing clinical trials in hospitals. -

ADAM GALLOWAY
Adam is an Advanced Practice Children’s Physiotherapist and a NIHR Clinical Doctoral Research Fellow in Leeds.
He has completed a PhD in Perthes’ Disease, focussing on the therapy-needs of children with Perthes’ Disease and their families.
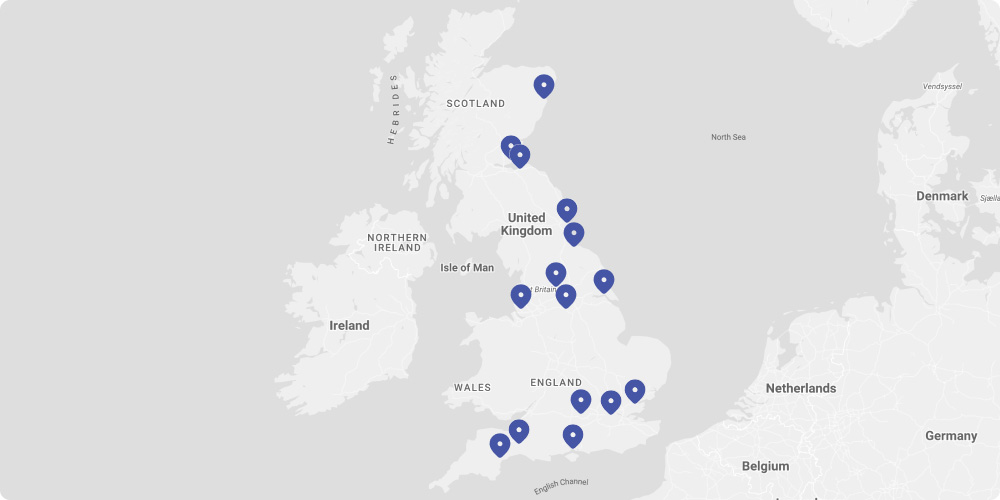
Study Sites
| Site/Hospital Name | Principal Investigator Name | Main Contact Name/Phone | PALS Phone/Email |
| Royal Berkshire Hospital | Nev Davies | Emma Gammin; Annie Warrington 01183228652 |
0118 322 8338 PALS@royalberkshire.nhs.uk |
| Alder Hey Children’s Hospital | Dan Perry | Natalie Copeland 07846298089 |
0151 252 5161 |
| Leeds Children’s Hospital | Adam Galloway | Adam Galloway 07708090305 |
0113 206 6261 patientexperience.leedsth@nhs.net |
| Sheffield Children’s Hospital | Sanjeev Madan | Nicolas Nicolaou 07801443331 |
0114 271 7594 scn-tr.pals@nhs.net |
| The James Cook University Hospital | Amanda Trees | Marc Atkinson; Joseph Nicholson 01642 850850 ext 52461 |
01642 854807 stees.patient.experience@nhs.net |
| Royal Devon and Exeter Hospital | Laura Tillotson | Evanna Brook 01392 406075 |
01392 402093 and 01271 314090 rduh.pals@nhs.net |
| Royal Aberdeen Children’s Hospital | Mike Reidy | Nicola Thomson 01224 551773 |
0800 917 2127 http://www.cas.org.uk/patientadvice |
| Hull Royal Infirmary | Sally Hobson | Laura Caley 01482 315506 |
01482623065 hyp-tr.pals.mailbox@nhs.net |
| St George’s Hospital | Yael Gelfer | Bethany Kenny bethany.kenny@stgeorges.nhs.uk |
020 8725 2453 pals@stgeorges.nhs.uk |
| University Hospital Southampton | Alex Aavold | Lorena Caruana Lorena.Caruana@uhs.nhs.uk |
02381 204 989 pals@uhs.nhs.uk |
| Victoria Hospital Kirkcaldy | Katie Hayes | Claire Stewart claire.stewart18@nhs.scot | 07554114913 fife.patientexperience@nhs.scot |
| Sunderland Royal Hospital | Dawn Lockey | Helen Griffith helen.griffith6@nhs.net | 0191 5656256 stsft.adviceandcomplaints@nhs.net |
| Royal National Orthopaedic Hospital | Neil Seragen | Rita Ochieng rita.ochieng@nhs.net | 020 8909 5439 / 5383. rnoh.pals@nhs.net |
| Musgrove Park Hospital | Becky Beamish | Kimberley Gillman research.generic@somersetft.nhs.uk | 01823 333444 https://www.somersetft.nhs.uk/contact-us/ |
| Royal Free Hospital | Olivia Malaga Shaw | Chidinma Iheanetu-Oguejiofor chidinma.iheanetu-oguejiofor@nhs.net |
020 8216 4924 rf-tr.bcfpals@nhs.net |
| Royal Hospital for Children & Young People | Emily Baird | Pamela Holland Pamela.Holland@nhs.scot |
0131 536 3370 LOTH.Feedback@nhs.scot |
| Broomfield Hospital | Pranai Buddhdev | Martina Vitaglione m.vitaglione@nhs.net |
01245 514130 mse.public.response@nhs.net |

